Background
Aplastic anemia (AA) is a rare but serious disease that affects hematopoietic stem cells and is characterized by pancytopenia and a hypocellular bone marrow. It can be a hereditary or acquired condition. Acquired AA has an incidence of 2 per million per year in Europe, but the incidence is two to three times higher in Asia. In Latin America, there is little epidemiologic data on this disease. The most important treatments for AA are bone marrow transplantation and immunosuppressive treatment with antithymocyte globulin and cyclosporine. But access to these treatments is restricted in some areas of Latin America.
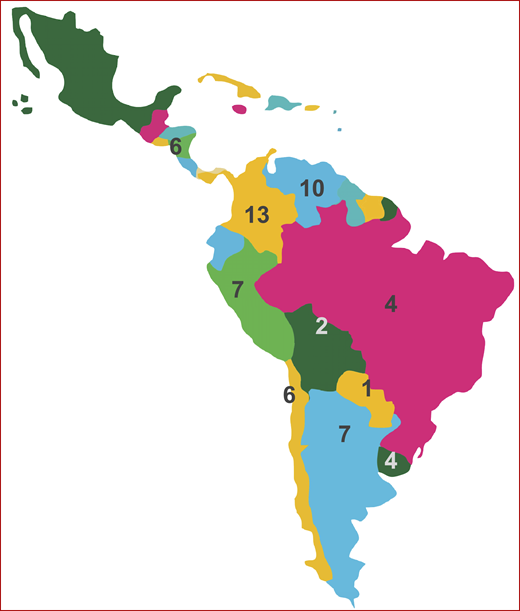
At the American Society of Hematology (ASH) Annual Meeting in 2016, representatives of the Hematology Societies of Latin America, with the support of the ASH International Program, met to discuss possible collaborative efforts. Everyone agreed that lack of reliable information is one of the main barriers to designing significant clinical trials for the region; therefore, starting a registry of hematologic diseases for the region has become a main goal of the group. In April 2017, at the ASH Highlights meeting in Latin America, AA was selected as the first disease that would be used to begin the collaborative action. National hematology societies of Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Paraguay, Peru, Uruguay, and Venezuela have made a commitment to help develop the Latin American Registry for Aplastic Anemia (LARAA) (Table 1). Figure 1 shows countries and the number of centers in each country that are likely to collaborate on building the registry.
Representatives of the hematology societies of Latin America collaborating in LARAA, by country
| Country . | Name of association or society . | Representatives . |
|---|---|---|
| Argentina | Sociedad Argentina de Hematología | Nora Patricia Watman,* Marcelo Iastrebner,* Dorotea Fantl, Luis Beligoy,* Vera Milovic* |
| Bolivia | Sociedad Boliviana de Hematologia y Hemoterapia | Juan Antonio Choque |
| Brazil | Associação Brasileira de Hematologia Hemoterapia e Terapia Celular | Rodrigo T. Calado* |
| Chile | Sociedad Chilena de Hematología | Vivianne Torres,* Ximena Huerta,* Gabriel Larroca,* Natalia Aránguiz,* Christine Rojas* |
| Colombia | Asociación Colombiana de Hematología y Oncología | Kenny Mauricio Gálvez,* Mónica Osuna Pérez,* Paola Omaña,* Carlos Pardo,* Claudia Sossa,* Virginia Abello |
| Costa Rica | Asociación Costarricense de Hematologia | Kathia Valverde |
| Paraguay | Sociedad Paraguaya de Hematología y Hemoterapia | José Ferreira Nizza, Víctor Salinas |
| Peru | Sociedad Peruana de Hematología | Rosa Vengoa,* Jaime Molina,* Pedro García,* Adriana Bustinza-Álvarez,* Alejandra LaTorre-Matuk,* Carolina Tokumura,* Juan Ramón Navarro,* Gabriela Vidal-Senmache* |
| Uruguay | Sociedad de Hematología del Uruguay | Carla Ambrosoni Balderrin,* María Victoria Irigoín,* Stefanía López,* Raúl Gabus* |
| Venezuela | Sociedad Venezolana de Hematología | Carlos Mendoza,* José Luis López* |
| Country . | Name of association or society . | Representatives . |
|---|---|---|
| Argentina | Sociedad Argentina de Hematología | Nora Patricia Watman,* Marcelo Iastrebner,* Dorotea Fantl, Luis Beligoy,* Vera Milovic* |
| Bolivia | Sociedad Boliviana de Hematologia y Hemoterapia | Juan Antonio Choque |
| Brazil | Associação Brasileira de Hematologia Hemoterapia e Terapia Celular | Rodrigo T. Calado* |
| Chile | Sociedad Chilena de Hematología | Vivianne Torres,* Ximena Huerta,* Gabriel Larroca,* Natalia Aránguiz,* Christine Rojas* |
| Colombia | Asociación Colombiana de Hematología y Oncología | Kenny Mauricio Gálvez,* Mónica Osuna Pérez,* Paola Omaña,* Carlos Pardo,* Claudia Sossa,* Virginia Abello |
| Costa Rica | Asociación Costarricense de Hematologia | Kathia Valverde |
| Paraguay | Sociedad Paraguaya de Hematología y Hemoterapia | José Ferreira Nizza, Víctor Salinas |
| Peru | Sociedad Peruana de Hematología | Rosa Vengoa,* Jaime Molina,* Pedro García,* Adriana Bustinza-Álvarez,* Alejandra LaTorre-Matuk,* Carolina Tokumura,* Juan Ramón Navarro,* Gabriela Vidal-Senmache* |
| Uruguay | Sociedad de Hematología del Uruguay | Carla Ambrosoni Balderrin,* María Victoria Irigoín,* Stefanía López,* Raúl Gabus* |
| Venezuela | Sociedad Venezolana de Hematología | Carlos Mendoza,* José Luis López* |
Patients retrospectively reported.
Latin American countries with LARAA participating centers and the number of centers in each country.
Latin American countries with LARAA participating centers and the number of centers in each country.
Objectives
Develop a multinational Latin American research group interested in studying AA in the region, its clinical characteristics, and therapeutic approaches;
Develop a Latin American Registry of patients with acquired AA to determine the current status of the disease in the region; and
Work together to improve diagnosis and treatment of AA by encouraging local action.
LARAA timeline
2016
December: ASH Annual Meeting in San Diego, CA. We agreed to design a collaborative effort across countries, to refine data collection efforts, and to start a collaborative registry project.
2017
April: At the Highlights of ASH meeting in Punta del Este, Uruguay, AA was selected as the first disease to begin collecting data to populate the registry.
June-October: Members of the work group filled out a survey on the state of AA in their countries. Table 2 describes the findings of the survey.
December: ASH Annual Meeting in Atlanta, GA. Representatives described the current state of AA diagnosis and treatment in their countries and the limitations they face. The group agreed to begin the first phase of the project by collecting 3 years’ worth of retrospective data (2016-2018) on AA in the region.
Diagnostic and treatment resources in Latin America
| Diagnostic resource . | No. of patients (N = 204) . | % . | Countries (N = 9) . |
|---|---|---|---|
| Bone marrow biopsy | |||
| Availability according to survey | 9 | ||
| Availability in preliminary data | 201 | 98 | |
| Paroxysmal nocturnal hemoglobinuria clones | |||
| Availability according to survey | 2 | ||
| Availability in preliminary data | 125 | 61 | |
| Cytogenetics | |||
| Availability according to survey | 3 | ||
| Availability in preliminary data | 92 | 45 | |
| Clastogenic effect of diepoxybutane | |||
| Availability according to survey | 3 | ||
| Availability in preliminary data | 68 | 33 | |
| Telomere length test | |||
| Availability according to survey | 1 | ||
| Availability in preliminary data | 52 | 25 | |
| Treatment availability | |||
| ATG/cyclosporine | 8 | ||
| Horse ATG | 2 | ||
| Stem cell transplantation | 8 | ||
| Unrelated stem cell transplantation | 3 | ||
| Eltrombopag for second-line treatment | 3 |
| Diagnostic resource . | No. of patients (N = 204) . | % . | Countries (N = 9) . |
|---|---|---|---|
| Bone marrow biopsy | |||
| Availability according to survey | 9 | ||
| Availability in preliminary data | 201 | 98 | |
| Paroxysmal nocturnal hemoglobinuria clones | |||
| Availability according to survey | 2 | ||
| Availability in preliminary data | 125 | 61 | |
| Cytogenetics | |||
| Availability according to survey | 3 | ||
| Availability in preliminary data | 92 | 45 | |
| Clastogenic effect of diepoxybutane | |||
| Availability according to survey | 3 | ||
| Availability in preliminary data | 68 | 33 | |
| Telomere length test | |||
| Availability according to survey | 1 | ||
| Availability in preliminary data | 52 | 25 | |
| Treatment availability | |||
| ATG/cyclosporine | 8 | ||
| Horse ATG | 2 | ||
| Stem cell transplantation | 8 | ||
| Unrelated stem cell transplantation | 3 | ||
| Eltrombopag for second-line treatment | 3 |
ATG, antithymocyte globulin.
2018
January-March: A subcommittee was created to work on a draft case report form (CRF) for data collection, and a protocol was developed.
April: At the Highlights of ASH meeting in Rio de Janeiro, Brazil, representatives presented preliminary data from their countries.
June-October: A national coordinator was designated for each country. A subcommittee was set up to finalize the CRF and the protocol.
December: ASH Annual Meeting in San Diego, CA. The study protocol and CRF were reviewed and approved at the meeting, with some modifications.
2019
January-March: A Spanish version of the protocol was updated, and final versions of the protocol and CRF were sent to the group. Country representatives began to submit materials for institutional review board (IRB) approval.
April: At the Highlights of ASH meeting in Lima, Peru, preliminary retrospective data were presented. The ASH Research Collaborative Data Hub was selected as the repository for the LARAA.
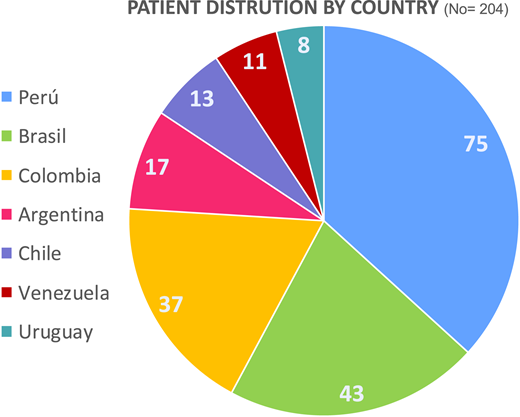
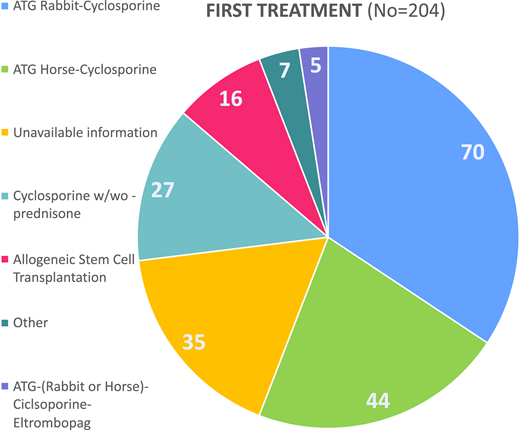
May-June: Preliminary retrospective data were collected. Figure 2 shows the distribution of patients by country. Table 3 shows retrospective cohort patient’s characteristics. Figure 3 represents the distribution of first-line treatments for the same group of patients.
LARAA preliminary results
| Demographic characteristics . | No. . | % . | Mean . | Range . |
|---|---|---|---|---|
| Total No. of patients | 204 | |||
| Age, y | 36.9 | 0.5-81.8 | ||
| Sex | ||||
| Female | 108 | |||
| Male | 96 | |||
| Diagnosis hemogram | ||||
| White blood cells, ×109/L | 2.5 | 0-8.5 | ||
| Hemoglobin, g/dL | 7.4 | 2.3-15.2 | ||
| Platelets, ×109/L | 20.5 | 0-204 | ||
| No. of transfusions before first treatment | ||||
| <10 | 90 | 44 | ||
| ≥10 | 96 | 46 | ||
| No. of exposures to toxins | ||||
| None | 137 | 67 | ||
| No data | 40 | 19.6 | ||
| At least 1 | 27 | 13 |
| Demographic characteristics . | No. . | % . | Mean . | Range . |
|---|---|---|---|---|
| Total No. of patients | 204 | |||
| Age, y | 36.9 | 0.5-81.8 | ||
| Sex | ||||
| Female | 108 | |||
| Male | 96 | |||
| Diagnosis hemogram | ||||
| White blood cells, ×109/L | 2.5 | 0-8.5 | ||
| Hemoglobin, g/dL | 7.4 | 2.3-15.2 | ||
| Platelets, ×109/L | 20.5 | 0-204 | ||
| No. of transfusions before first treatment | ||||
| <10 | 90 | 44 | ||
| ≥10 | 96 | 46 | ||
| No. of exposures to toxins | ||||
| None | 137 | 67 | ||
| No data | 40 | 19.6 | ||
| At least 1 | 27 | 13 |
Next steps
Complete IRB approvals,
Finish retrospective data collection and prepare it for publication,
Design a digital CRF (eCRF) and protocols for prospective studies,
Collect and publish prospective data, and
Define actions for improving diagnostic tools and treatment in the region.
Summary
Latin America has great potential for good-quality research in hematology, but multiple barriers have made this goal difficult to achieve until now. One of the barriers is the lack of reliable regional information on disease epidemiology, patient characteristics, access to treatment, and results, all of which are necessary to design relevant trials and resolve local problems. National hematology associations are key to bringing together researchers and optimizing efforts to achieve success in all of their projects. Once the willingness to work together was manifested by multiple Latin American hematology associations, creating a registry on hematologic diseases became the main objective. AA was selected as the first disease to focus on. LARAA reflects determination to work together for a common goal. The initial results show that all of the countries involved have many similarities and also many differences. Our goal is to make an initial assessment of where we stand, and to propose solutions to the problems of access to diagnosis and appropriate treatment for patients with AA in the region.
Acknowledgment
The authors thank Michelle Lara, International Programs Manager, Executive Office, American Society of Hematology.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginia Abello, Fundación Universitaria de Ciencias de la Salud, Facultad de Medicina, Calle 10 No. 18-75, Bogotá, Colombia; e-mail: virginia.abello@gmail.com.